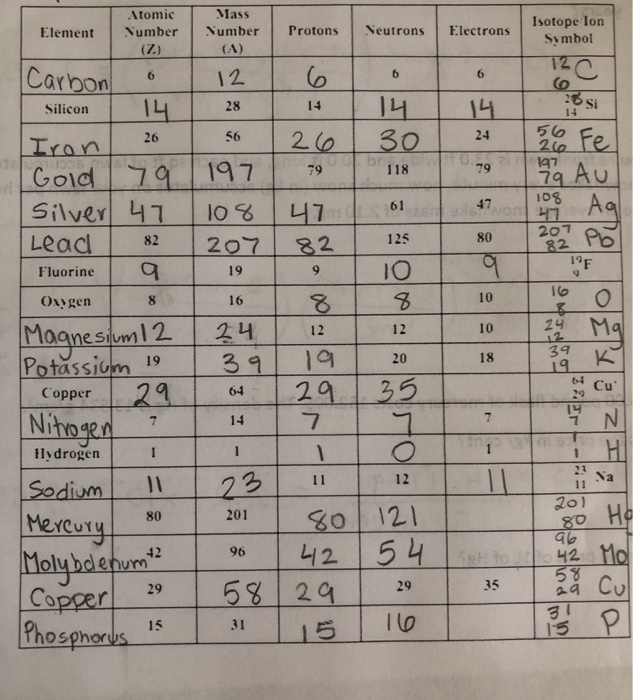
The most common version is C-12 6p 6n 6e whose mass of 12amu comes from the 6 protons and 6 neutronsThe balance of 6 protons and 6 electrons makes the atom electrically neutral no charge. In this video well use the Periodic table and a few simple rules to find the protons electrons and neutrons for the element Fluorine F.
Protons Neutrons Electrons And Isotopes
The most common version is C-12 6p 6n 6e whose mass of 12amu comes from the 6 protons and 6 neutronsThe balance of 6 protons and 6 electrons makes the atom electrically neutral no chargeRelated searches for fluorine protons neutrons electronsnombre protons neutrons et lectronstrouver le nombre de protonsschma proton neutronnombre de protons et dlectronscomment calculer des protonscalcul neutrons protons lectronsoxygne nombre de neutronscomment trouver le nombre dlectronsSome results have been removedPagination12345NextSee moreSee all imagesFluorineFluorine is a chemical element with the symbol F and atomic number 9.
Fluorine protons neutrons electrons. Will an atom with 6 protons 6 neutrons and 6 electrons be electrically neutral. Meanwhile its mass number of 19 minus 10 neutrons gives you 9 protons or electrons. Protons Neutrons and Electrons of all the Elements Shell Diagram 1 Hydrogen has 1 proton 0 neutron and 1 electron2 Helium has 2 protons 2 neutrons and 2 electrons3 Lithium has 3 protons 4 neutrons and 3 electrons.
Learn vocabulary terms and more with flashcards games and other study tools. Isotopes have the same number of protons and electrons but a different number of neutrons. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral.
ThanksGive more feedback See results forNeutron050James Chadwick Ernest R Isotope8thICI 2014 PIM 2013Frederick Soddy Joseph Jo. Learn vocabulary terms and more with flashcards games and other study tools. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas.
The total electrical charge of the nucleus is therefore Ze where e. Were always here. It says 9 on fluorine on the Periodic Table so Im wondering if that means fluorine has 9 neutrons 9 electrons and 9 protons.
ReadWhat is the number of protons in fluorine. In this video well use the Periodic table and a few simple rules to find the number of protons and electrons for the Fluoride ion F-. A fluorine atom is larger than a carbon atom because fluorine has more protons and more electrons than carbon.
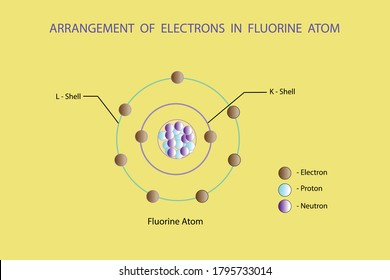
Protons and neutrons are located in the nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The atomic number is the number of protons.
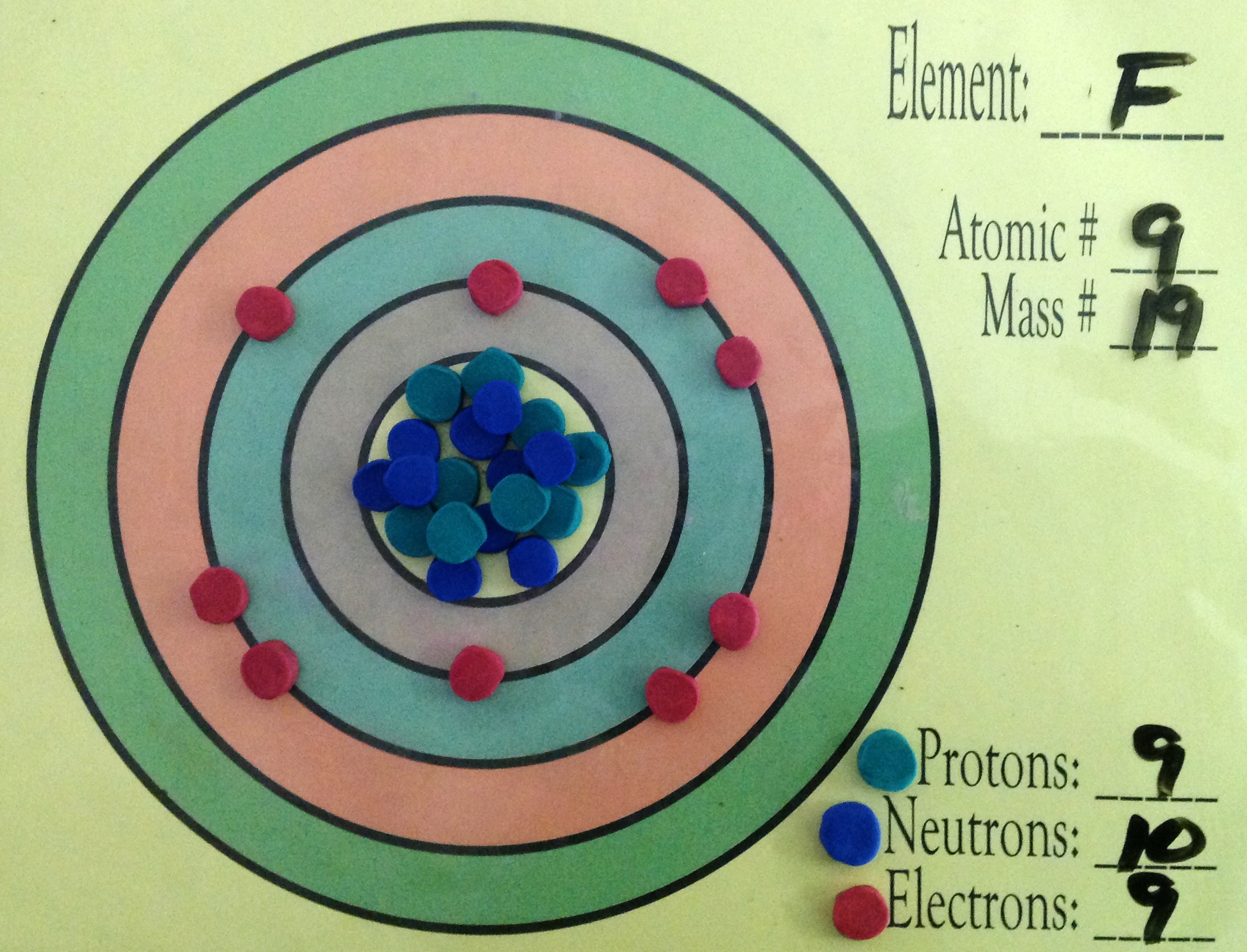
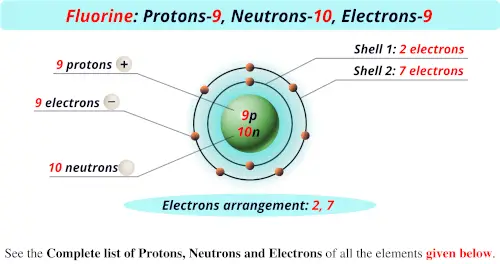
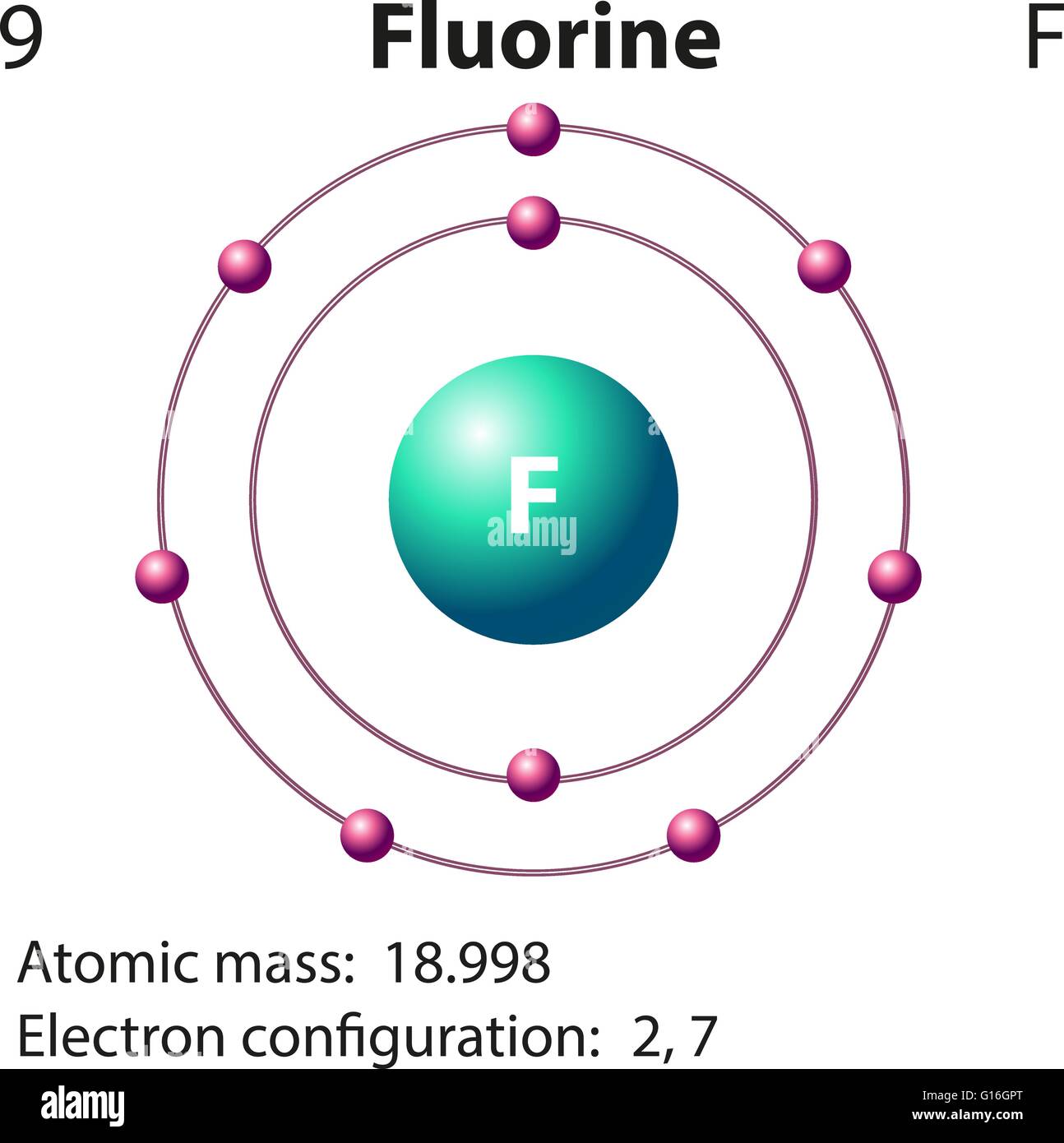
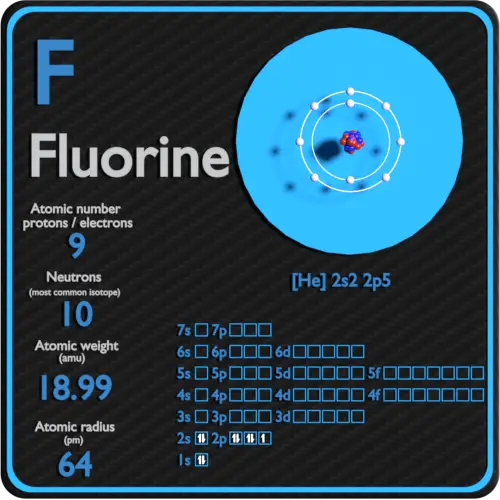
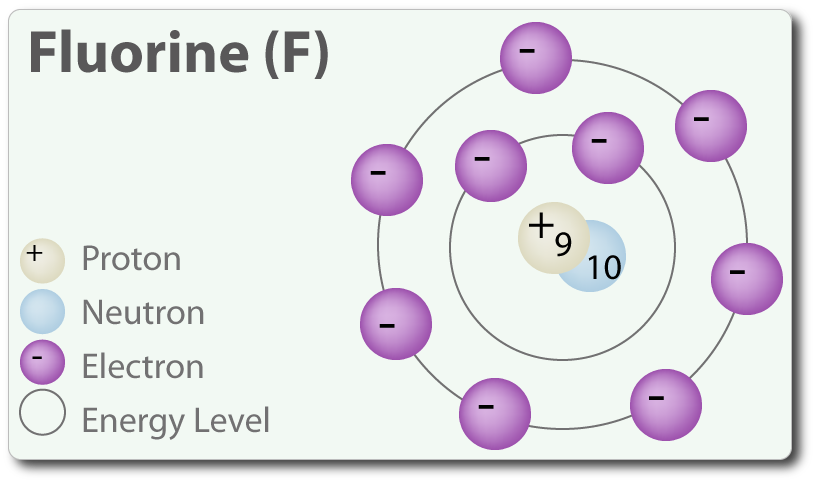
That is the number of protons in the fluorineF is 9. Identify Fluorine Atomic Mass 18998 atomic mass models Variety of Protons 9 Variety of Neutrons 10 Variety of Electrons 9 Click on to see full reply Additionally how many electrons does fluorine have. Hence the atom would be Fluorine.
Electrons equal to protons are located in a. Answer 1 of 4. 5348 K 90 kPaBenefitsAnother benefit of fluorine is that it can be used to help molten metal flow.
Home Subjects Explanations Create Study sets textbooks Log in. The atomic number of fluorineF is 9. Calculate the number of protons neutrons and electrons in fluorine-19.
9 The element fluorine helps prevent tooth decay and is found in many toothpastes. Finally Which of. F 8503 K 18811 C 30660 FDensity at STP.
A common exam question is to describe the difference between two isotopes. 1696 gLwhen liquid at bp. 18998 atomic mass units.
Fluorine has an electron configuration of He 2s2 2p5 and is usually found as a stable diatomic molecule by forming a single covalent bond between two F atoms. If a fluorine atom gains an electron it becomes a fluoride ion with an electric charge of -1. Will an atom with 6 protons 6 neutrons and 6 electrons be electrically neutral.
Asked Sep 1 2019 in Chemistry by Laurie general-chemistry. A neutral atom has the same number ofThe nucleus of a fluorine-19 atom contains A. This preview shows page 1 - 4 out of 7 pages.
So for the element of FLUORINE you already know that the atomic number tells you the number of electrons. How many electrons and protons does fluorine have. Answer 1 of 4.
The atomic number of fluorineF is 9. Atomic Number Protons Electrons and Neutrons in Fluorine. F 5348 K 21967 C 36341 FBoiling point.
Pure fluorine is so reactive with other materials that it is never found in that form in natureit is always chemically bound to other materials. 9 ElectronHow many neutrons does fluorine have20160820How many protons neutrons electrons are in 18F-. So we have 42 protons and if its neutral we have 42 electrons and then well have 96 minus 42 or 54 neutrons.
This is actually where the name comes from. Were always here. So for the aspect of FLUORINE you already know that the atomic quantity tells you the variety of electrons.
Name Fluorine Atomic Mass 18998 atomic mass units Number of Protons 9 Number of Neutrons 10 Number of Electrons 9 Also how many electrons does fluorine have. Since the number of electrons equal the number of protons Fluorine has 9 electrons as well. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.
Fluorine is a chemical element with the symbol F and atomic number 9. Meanwhile its mass number of 19 minus 10 neutrons gives you 9 protons or electrons. It is very useful in situations where molten metal must not stand still but cannot move on its own.
Of the unstable nuclides of fluorine 18F has the longest half-life 109739 minutes. Fluorine-18 is composed of 9 protons 9 neutrons and 9 electrons. Start studying Protons Electrons and Neutrons - l FREED WITH ANSWERS.
Identify Fluorine Atomic Mass 18998 atomic mass models Variety of Protons 9 Variety of Neutrons 10 Variety of Electrons 9 Click on to see full reply Additionally how many electrons does fluorine have. 53 The element iodine is often used to clean skin before surgery. WikipediaWikipedia text under CC-BY-SA licenseWas this helpful.
For this reason 18F is a commercially important source of positrons. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. How many protons are in the nucleus of F Name Fluorine Number of Protons 9 Number of Neutrons 10 Number of Electrons 9 Melting Point -21962 C Furthermore How many protons and electrons are in the f ion A fluorine atom has nine protons and nine electrons so it is electrically neutral.
9 The element fluorine helps prevent tooth decay and is found in many toothpastes. Fluorine Protons Neutrons Electrons Electron Configuration Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. By looking at the periodic table you will see that Fluorine has 9 protons.
So we have 42 protons and if its neutral we have 42 electrons and then well have 96 minus 42 or 54 neutrons. Identify the number of protons and electrons in fluorine and iodine. That is the number of protons in the fluorineF is 9.
All isotopes of fluorine have nine protons. Fluorine-20 is composed of 9 protons 11 neutrons and 9. As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and heliumWikipedia Phase at STP.
9 is thus its atomic number and identity as an element. How many electrons and protons does fluorine have. As the most electronegative element it is extremely reactive as it reacts with all other elements except for.
A neutral atom has the same number of. As the most electronegative element it is extremely reactive. Fluorine has an electron configuration of He 2s2 2p5 and is usually found as a stable diatomic molecule by forming a single covalent bond between two F atoms.
By looking at the periodic table you will see that Fluorine has 9 protons. A fluorine atom is larger than a carbon atom because fluorine has more protons and more electrons than carbon. Join our Discord to connect with other students 247 any time night or day.
53 The element iodine is often used to clean skin before surgeryProtons Neutrons Electrons and Isotopeshttpswwwchemistrygcsecouk1 - Core ChemistryAtoms. This preview shows page 1 - 4 out of 7 pages. How many protons neutrons and electrons are in fluoride.
It has two decay modes of which the main one is positron emission. Since the number of electrons equal the number of protons Fluorine has 9 electrons as well. Start studying Protons Electrons and Neutrons - l FREED WITH ANSWERS.
The atomic number is the number of protons. Identify the number of protons and electrons in fluorine and iodine. Almost all other elements including some noble gases form compounds with fluorine.
So for Oxygen-18 and Oxygen-16 you should write that they both have 8 protons and 8 electrons but Oxygen-18 has 10 neutrons while Oxygen-16 had 8 neutrons. From the Periodic Ta. 18998 atomic mass units.
Considering this how many electrons protons and neutrons. Electrons equal to protons are located in a How many electrons and protons does fluorine have. A fluorine atom has nine protons and nine electrons so it is electrically neutral.
So for the aspect of FLUORINE you already know that the atomic quantity tells you the variety of electrons. A common exam question is to describe the difference between two isotopes. Fluoride Protons Neutrons Electrons Fluorine-20 Protons Neutrons Electrons Name.
Isotopes have the same number of protons and electrons but a different number of neutrons. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. Fluorine - 23 has 9 protons 9 electrons and 14 neutrons.
Solved Atomic Number Element Mass Sumber A Protons Chegg Com
Protons Neutrons Electrons Of All Elements List Images

3d Render Of Atom Structure Of Fluorine Isolated Over White Background Protons Are Represented As Red Spheres Neutron As Yellow Spheres Electrons As Stock Photo Alamy

9 F Fluorine Electron Shell Structure Schoolmykids Electron Configuration Periodic Table Atomic Structure

See The Electron Configuration Of Atoms Of The Elements Fluorine Atom Electron Shell Diagram Electron Configuration Atom Diagram Atom Project
Fluorine Atomic Structure Stock Image C018 3690 Science Photo Library

Blog Archive Bohr Model Of Fluorine Atom With Proton Neutron And Electron Chanhassen Family Dentistry

Dublin Schools Lesson Ions How Do The Number Of Subatomic Particles Differ For Atoms With A Charge
Chemical Elements Com Fluorine F

How To Find The Number Of Protons Electrons Neutrons For Fluorine F Youtube

Identify How Elements Are Arranged On The Periodic Table F Fluorine Atu 9 How Many Particles In The Nucleus Protons Neutrons Electrons Now Ppt Download

How Many Unpaired Electrons In A Ground State Fluorine Atom Socratic
Drawing Atoms Protons Neutrons And Electrons By Chemistry Wiz
Fluorine Atom Bohr Model With Proton Neutron And Electron Stock Photo Download Image Now Istock
Fluorine Element High Resolution Stock Photography And Images Alamy
Fluorine Protons Neutrons Electrons Electron Configuration
Fluorine Atom High Res Stock Images Shutterstock
Chem4kids Com Fluorine Orbital And Bonding Info



Post a Comment
Post a Comment